5 Blood Alcohol Concentration
Introduction
Purpose
Quantify the amount of ethanol in an unknown ‘blood’ sample using headspace GC with a flame ionization detector.
BAC Samples
Blood alcohol measurement is one of the most common toxicology analyses performed in the U.S. In addition to breath alcohol testing with preliminary breath testing instruments and/or IR, GC-FID (flame ionization detector) of blood samples is the typical way we do this. Urine can be tested for ethanol metabolites, but it is generally less reliable.
Headspace sampling is the typical method used for GC sample introduction in BAC analyses, as shown in Figure 1 below. It is performed by placing a blood sample in a headspace vial. Headspace vials are sealed but have a septum that may be pierced by a syringe. By gently heating the vials, we can take advantage of the partial pressure of the gas phase ethanol being proportional to the concentration of ethanol in the blood sample. A gas-tight syringe can then be used to extract a headspace sample containing gas phase ethanol and inject the sample onto the GC column.
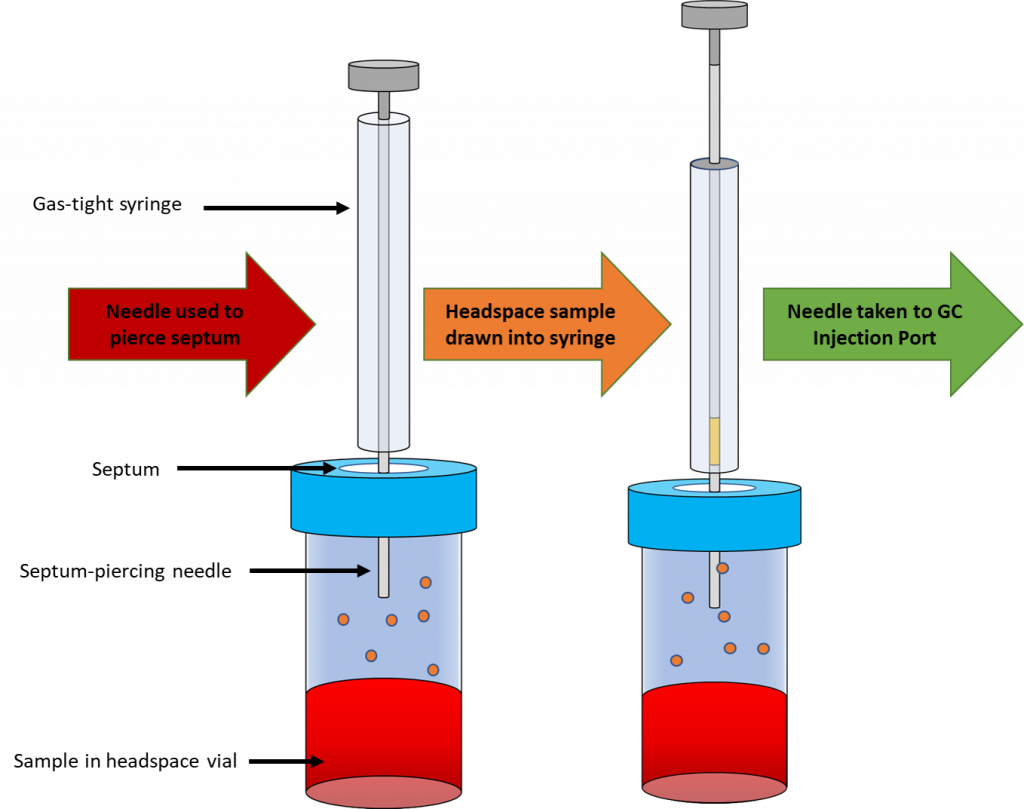
Materials and Methods
Supplies
- Ethanol
- Internal standard
- Volumetric flask(s)
- Pipette
- Unknown samples
- Headspace vials
- Headspace-GC-FID
Procedure
Prepare Calibration Solutions
- Determine the ethanol concentrations you want to use for external calibration and have them approved by the TA
- Be sure to take a 1:1 dilution with the internal standard solution into account
- Make your calibration solutions
- Transfer 1 mL aliquots to a headspace vial and label it with the concentration
- Add 1 mL of internal standard solution to the vial
Prepare Unknown Sample
- Transfer 1 mL of your sample to a headspace vial and label it
- Add 1.0 mL of internal standard solution to the unknown in the vial
GC-FID
- First, run a blank
- Run calibration solutions in triplicate
- Check that all RTs, ethanol and internal standard, are acceptable
- With your TA, ensure the data is acceptably linear using the provided software
- R-squared = 0.95-0.985 is typically the minimum desired linearity
- You may move forward with unknowns if you obtain a value of 0.80 or higher if all but 1 data point seem reasonably linear
- If your R-squared value is between 0.80-0.95, perform a Grubb’s Test when writing your report and before calculating your unknown concentration
- R-squared = 0.95-0.985 is typically the minimum desired linearity
- Each group should then run their unknown sample in triplicate
- Ensure your internal standard is present and RTs are acceptable
- Each group should use the software to obtain peak areas of the calibration and unknown samples
- Obtain data for one calibration chromatogram and the unknown chromatogram to plot
Lab Report
- Data table with concentrations and areas
- Calibration curve and linear regression equation
- Grubb’s Test calculations and if your R-squared value is less than 0.95
- Original and revised calibration curve, regression equation, and R-squared if you remove an outlier based on Grubb’s Test results
- How did removing the point affect your data?
- Calculate concentration of unknown with original and revised calibration curves
- How did removing the point affect your final results?
Media Attributions
- BAC Headspace Sampling © Charlie Williams

